Versatile reactors for light-induced molecular transformation
Recent studies in the UHV suggest a new mechanism for the photocatalytic conversion of alcohols on Pt-decorated rutile TiO2(110) single crystals. To elucidate the transferability of this mechanistic concept, we developed versatile gas and liquid phase photoreactors. The findings we obtained provide understanding that is pivotal for the tailored development of new efficient photocatalysts.

Focus Group: Light-Induced Molecular Transformations
on Cluster-Assembled Materials
Prof. Ib Chorkendorff (Technical University of Denmark), Alumnus Hans Fischer Senior Fellow | Clara Aletsee, Charitini Panagiotopoulou, Carina Schramm (TUM), Doctoral Candidates | Hosts: Prof. Ueli Heiz, Prof. Ian Sharp (TUM)
Heterogeneous photocatalytic systems are usually described on the basis of electrochemistry, which most interpretations and strategies for optimizing photocatalysts rely on. Charge carrier dynamics are usually in the spotlight, whereas the surface chemistry of the photocatalyst is neglected. This is unjustified, because studies on alcohol photoreforming on metal-decorated rutile single crystals revealed the electrochemical reaction model not to be generally applicable. Hence, many photocatalytic reactions may proceed in a different manner, and the thermal chemistry needs to be accounted for. Combining a variety of surface science techniques, we found a new surface reaction mechanism, illustrated in Fig. 1. Upon dissociative adsorption of the alcohol, a surface-bond hydrogen and the corresponding alkoxy species are thermally formed. The latter is suggested to interact with the negatively charged defect states of the titania surface [1]. Being the photoactive species, the alkoxy species reacts in a photo-hole mediated step, in which the C-H bond is homolytically cleaved. This forms the corresponding aldehyde or ketone, which thermally desorbs from the surface.
Figure 1
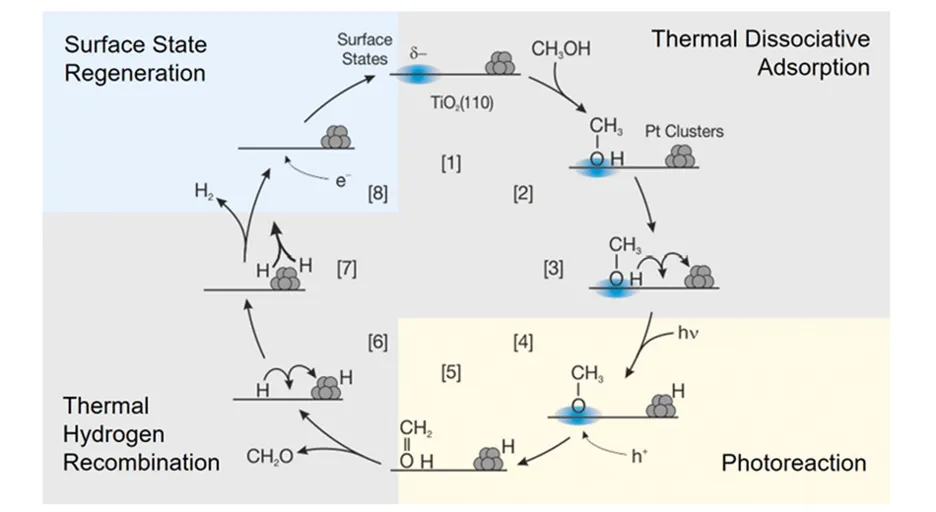
The evaluation of this new reaction mechanism under more applied conditions, such as, e.g., ambient pressure or liquid phase, and for materials other the single crystals, has been challenging since fabrication of such model photocatalysts often involves tedious strategies, yielding only low quantities in the μ-gram scale. In addition, these model catalysts exhibit different forms, such as powders or film(-like) structures grown on various supporting materials. Therefore, in the first part of this project, we introduced a new design concept for a gas phase μ-photoreactor [2] as shown in Fig. 2. It is tailored not only to host low catalyst amounts of different shapes, such as in planar form or as powders, but also to be re-openable, which enables a fast sample exchange for screening studies and analysis of the catalyst material after the reaction experiments, mandatory for obtaining details on the reaction mechanism. Briefly, the reactor is based on a fully Pyrex-based lid with an implemented gas flow channel system, which is pressed on the supported catalyst by a polymeric spacer with a circular cutout, creating a reactor chamber volume of only 10.5 μl. The entire gas flow is transmitted by a capillary to a QMS, enabling time-resolved and sensitive reaction monitoring at ambient pressure.
The lid fabrication from two Pyrex wafers by a lithographic and wet-etching approach provides capillaries in the desired, reproducible μm-dimensions. The consecutive fusing strategy allows illumination with an interchangeable light source (λ > 320 nm).
The gas-dependent flow rate through the capillary was determined to be 1015–1016 molecules s−1. These values corroborated first estimations using pump speeds and obtained pressures in the QMS.
Figure 2
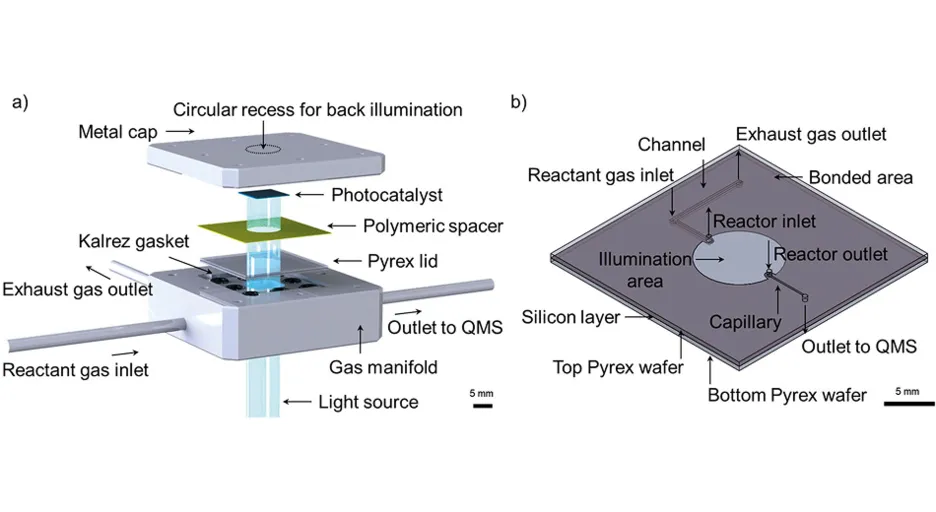
The functionality of the device was demonstrated by the photooxidative dehydrogenation of ethanol over Pt-loaded TiO2 (P25) at varying illumination intensities. By means of dark-illumination difference spectra, reaction products were unambiguously identified despite the presence of several overlapping mass peaks in the QMS. A version of this photoreactor was also made that is compatible with a commercial setup for dark electrochemical reactions [3].
For the evaluation of photocatalytic materials in the liquid environment, we also designed a liquid phase reactor. The reactor itself is exchangeable, which allows the characterization of a variety of catalysts with respect to shape (e.g., powder, planar structures) and extends the usability of the setup toward other applications such as electro(-photo)catalysis.
These versatile reactor concepts have been proven to be well suited for screening photocatalytic materials under various chemical environments and with various shapes, which are available only in small amounts due to their time-consuming fabrication. Furthermore, changes resulting in the catalyst during the reaction can be studied by opening the reactor and characterizing the material afterwards. The combination of these properties has proven beneficial to enable studies of photocatalytic reactions or materials.
Figure 3
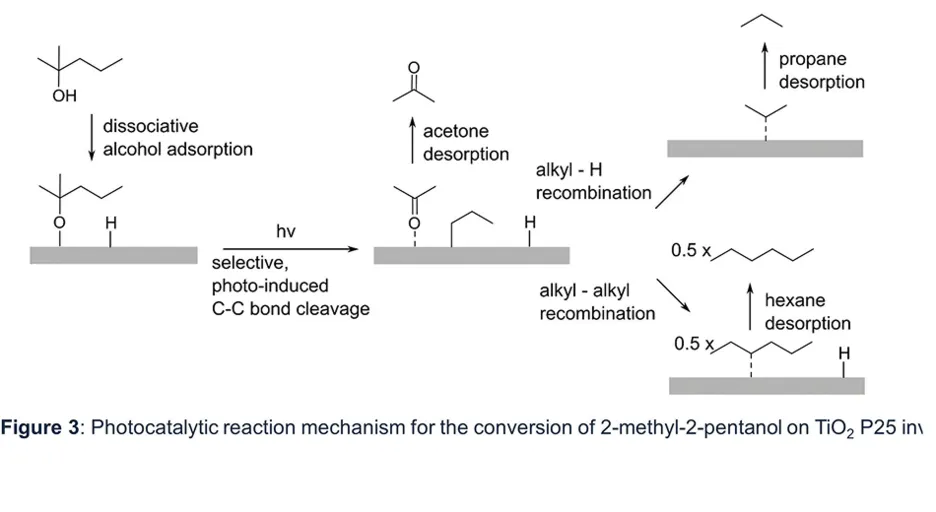
To illustrate the versatility of these reactors, we investigated the selective photooxidation of tertiary alcohols at ambient conditions (1000 mbar, T = 23°C) in an anaerobic environment on bare TiO2 P25 upon UV irradiation [4]. Exemplified for 2-methyl-2-pentanol and 2-methyl-2-butanol, the reaction proceeds exclusively via the photo-induced, homolytic cleavage of the long alkyl chain, resulting in the respective ketone and the alkyl-moiety, which predominantly recombines with hydrogen upon alkane formation. Alternatively, the dimerization of two alkyl-radicals occurs as a side reaction and happens on bare TiO2 at enhanced alcohol surface concentrations. The reaction scheme is shown in Fig. 3. This demonstrates that alkyl-radical chemistry is enabled on bare TiO2, which is also relevant for other reactions, such as Kolbe-type ones.
These new reactor platforms open an avenue for fast and versatile testing of potential photocatalysts in the search for new processes with high selectivity that is not accessible by conventional thermal processes.
[1]
Walenta, C.A. et al. (2019).
[2]
Aletsee, C.C. et al. (2023).
[3]
Hochfilzer, D. et al. (2024).
[4]
Aletsee, C.C. et al. (2025).
Selected publications
- Walenta, C.A., Tschurl, M. & Heiz, U. Introducing catalysis in photocatalysis: What can be understood from surface science studies of alcohol photoreforming on TiO2. J. of Physics: Condensed Matter 31(47), 473002 (2019).
- Aletsee, C.C., Hochfilzer, D., Kwiatkowski, A., Becherer, M., Kibsgaard, J., Chorkendorff, I., Tschurl, M. & Heiz, U. A re-useable microreactor for dynamic and sensitive photocatalytic measurements: Exemplified by the photoconversion of ethanol on Pt-loaded titania P25. Rev. Sci. Instrum. 94(3), 033909 (2023).
- Hochfilzer, D., Aletsee, C.C., Krempl, K., Pedersen, T., Krabbe, A., Tschurl, M., Hansen, O., Vesborg, P.C.K., Kibsgaard, J., Heiz, U. & Chorkendorff, I. Enabling real-time detection of photocatalytic reactions by a re-useable µ-reactor. Measurement Science and Technology 35(1), 015903 (2024).
- Aletsee, C.C., Neumann, P., Chorkendorff, I., Tschurl, M., & Heiz, U. Tertiary alcohols as mechanistic probes for photocatalysis: the gas phase reaction of 2-pethyl-2-pentanol on Titania P25 in a Micro-Photoreactor, ACS Catalysis 15(3), 2584-2594 (2025).