Maternal stress affects the child neurocognitive and epigenetic outcome
Prenatal exposure to maternal psychosocial stress confers a lifelong risk for behavioral alterations that last beyond childhood. Our aim is to identify non-invasive biomarkers of the childdevelopmental outcome to offer a precise and truly personalized prediction and new possibilities for designing interventions to improve neurodevelopmental outcomes of pregnancy affected by prenatal stress.

Focus Group Early Biomarkers of Prenatal Stress
Prof. Marta C. Antonelli (University of Buenos Aires), Alumna Hans Fischer Senior Fellow | Ritika Sharma (TUM), Doctoral Candidate | María Sol Molinet (TUM), Dr. med. Candidate | Hosts: Prof. Marion Kiechle, PD Dr. Silvia M. Lobmaier (Women’s Clinic at TUM University Hospital rechts der Isar)
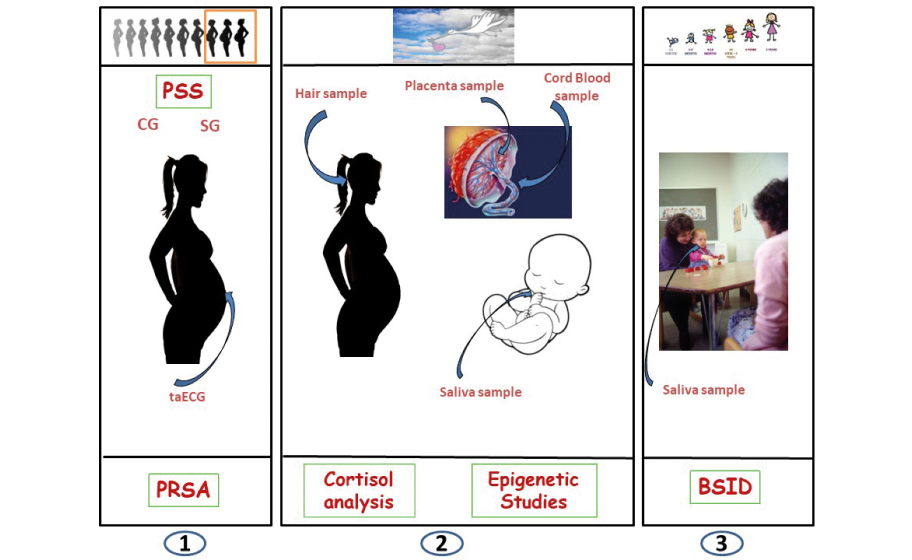
Pregnancy is a significant time in women’s lives but it can also be very challenging. During the gestational period, women like any other subject, can be exposed to endogenous and exogenous challenges that may be perceived as unpleasant, aversive or threatening in such a way that the homeostasis, wellbeing and overall health is threatened. This means that maternal stress during pregnancy and during early parenting can program physiological responses and lifetime trajectories of the infant, which in interaction with genetic liabilities and early-life challenges, will determine ultimate health status. Our main objective is to test the feasibility of identifying early non-invasive pre- and postnatal biomarkers of brain programming due to intrauterine stress exposure. To this end, we performed a prospective matched control study in stressed mothers with controls matched for parity, maternal age, and gestational age at study entry. Subjects were recruited for 36 months from a cohort of pregnant women followed in the Department of Obstetrics and Gynecology at “Klinikum rechts der Isar” of the Technical University of Munich (TUM). Maternal psychosocial stress was measured using the Cohen Perceived Stress Scale (PSS 10) to measure nonspecific perceived stress and was classified into stressed (SG) and control group (CG). Prenatal Distress Questionnaire (PDQ) was also administered to assess specific pregnancy worries. Two and a half weeks after screening, we performed a transabdominal ECG (taECG). Upon delivery, maternal hair strands were collected for cortisol measurements and newborns’ saliva and cord blood samples were collected. Two years after delivery, infants’ cognitive, language and motor development were assessed by Bayley Scale III of Infant Development (BSID) (Figure 1).
Figure 1
Main achievements
In our quest for finding a prenatal measure that might have a preventive clinical significance we hypothesize that the coordinated roles of the ANS (Autonomic Nervous System) and the HPA (Hypothalamic Piuitary Axis) in the integrated stress response can be monitored non-invasively using electrocardiogram (ECG) and ECG-derived maternal and fetal heart rate (mHR, fHR). We propose a novel analysis method of coupling between mHR and fHR based on a signal-processing algorithm, first applied in adult cardiology, termed bivariate phase-rectified signal averaging (BPRSA) and applied to trans-abdominally acquired fetal ECG (fECG). Our first results show that PSS-10 shows a correlation with the coordination of fetal and maternal heart rate and fetal oxygenation at birth. The proposed BPRSA index (FSI) provides unique insights into the relationship between two biological systems: mother and fetus. Interestingly, CG fetuses remained “stable” during these periods whereas fetuses of stressed mothers showed significant decreases of fHR. [1]
Our next goal was to quantify the levels of methylation across this entire genome in the newborn saliva employing EWAS (Epigenome-Wide Association Study) as a biomarker detector of epigenetic reprogramming in young infants. DNA was extracted from saliva samples and DNA methylation was measured using EPIC Bead-Chip array (850k CpG sites). To identify associations between PSS PDQ / FSI / Cortisol and methylation, linear regression models adjusting for confounders were run. We found epigenome-wide significant associations for 6 CpG sites in association with stress phenotypes (PDQ and cortisol). Annotated genes to the CpGs were found to be enriched for development and growth in the hippo signaling pathway, organelle biosynthesis, and metabolism of proteins, cell proliferation and bone development. We report novel associations between DNA methylation and maternal stress phenotypes. We observe that the several associations are related to neurobiological disorders such as autism, schizophrenia, neural tube defects and atrophy [2]. Since this is a longitudinal study, we are assessing the neurodevelopmental outcome of the two years old infants and we will assess the differential methylation in saliva samples at that age. Detecting differences in methylation between the two ages will give a window of opportunity for early interventions.
Figure 2
Matching the rising need for iron during pregnancy is important to prevent an impairment of the growing child’s neurodevelopment due to iron deficiency. Our next objective was to assess the influence of prenatal maternal stress (PS) on the neonatal iron homeostasis. Neonatal cord blood serum hepcidin, transferrin, and iron were determined. Transferrin saturation and iron were lower in male stressed neonates. SG reduces neonatal ferritin by 15.4 % compared to controls. PS during the third trimester perturbs neonatal iron markers in a sex-dependent manner [3]. Early detection of PS can support early individualized pre- and postnatal iron supplementation and neurodevelopmental follow-up to prevent long-term sequelae.
As mentioned before, chronic prenatal stress results in entrainment of the fetal heartbeat by the maternal heartbeat, quantified by the fetal stress index (FSI). Deep learning (DL) is capable of pattern detection in complex medical data with high accuracy in noisy real-life environments, but little is known about DL’s utility in non-invasive biometric monitoring during pregnancy. A recently established self-supervised learning (SSL) approach to DL provides emotional recognition from electrocardiogram (ECG).
In collaboration with Dr. Martin Frasch (University of Washington, Seattle, USA) we hypothesized that SSL will identify chronically stressed mother-fetus dyads from the raw maternal abdominal electrocardiograms (aECG), containing fetal and maternal ECG. Our DL models accurately detect the chronic stress exposure group, the individual psychological stress score and FSI at 34 weeks of gestation, as well as the maternal hair cortisol at birth reflecting chronic stress exposure [4]. The final DL model can be deployed in low-cost regular ECG biosensors as a simple, ubiquitous early stress detection and monitoring tool during pregnancy. This discovery should enable early behavioral interventions.
Two years after delivery, infants’ cognitive, language and motor development were assessed by Bayley Scale III of Infant Development (BSID). Cognitive and motor areas showed no significant variation between SG and CG toddlers even when accounting for sex. However, language composite scores showed a significant decrease in SG toddlers irrespective of sex and language spoken at home. When day care center attendance (DCA) was accounted for, this effect disappeared. PS affects the toddlers’ language development in both sexes, regardless the language spoken at home. DCA seems to protect for this neurodevelopmental delay. These results confirm the importance of early stimulation through social interaction for reversion of language delays in toddlers exposed to PS.
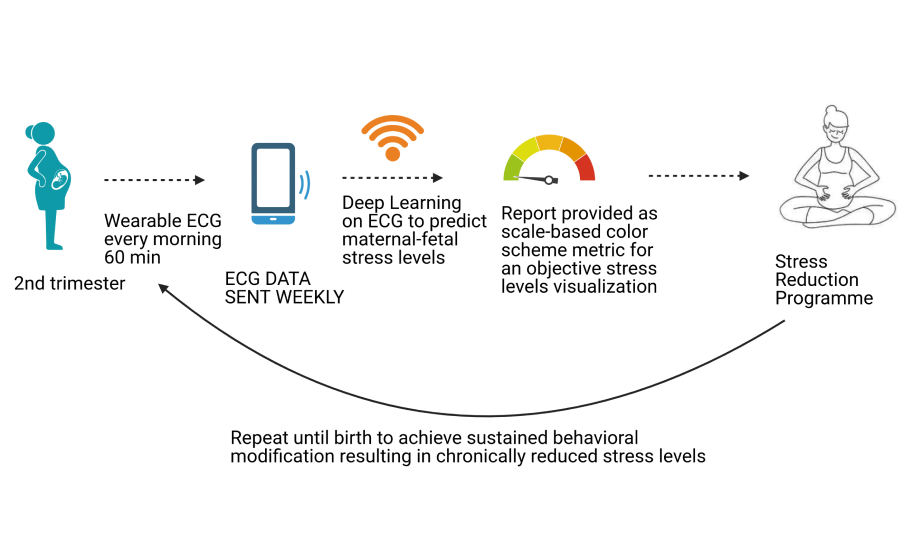
In summary, we discovered how to detect the impact of chronic stress on mother and child during pregnancy non-invasively using several biomarkers combined with artificial intelligence techniques that could be employed as predictive biomarkers of the child neurodevelopmental outcome [5]. However, we believe that the best strategy lies in the preventive nature of the interventions that reduce stress during pregnancy and potentially avoid negative child outcomes. Based on our previous findings, we are now developing a project that brings together early detection and intervention yoga / mindfulness programs in a digital biofeedback paradigm compatible with today’s challenges of pandemic and telemedicine (Figure 2). Our approach might lead to at-scale adoption of prevention of the detrimental effects of chronic stress on the child’s potential.
In close collaboration with Dr. med. Camilla Zelgert, Dr. med. Peter Zimmermann (TUM).
[1]
Lobmaier, S. M. et al. (2020).
[2]
Sharma, R. et al. (2022).
[3]
Zimmermann, P. et al. (2022).
[4]
Sarkar, P. et al. (2022).
[5]
Antonelli, M.C. et al. (2022).
Selected publications
-
Lobmaier, S. M. et al. Fetal heart rate variability responsiveness to maternal stress, non-invasively detected from maternal transabdominal ECG. Archives of Gynecology & Obstetrics 301(2), 405–414 (2020), doi.org/10.1007/s00404-019-05390-8.
-
Sharma, R. et al. Maternal-Fetal stress and DNA methylation signatures in neonatal saliva: An Epigenome-Wide Association Study. Clinical Epigenetics 14, 87 (2022), doi.org/10.1186/s13148-022-01310-x.
-
Zimmermann, P. et al. Prenatal stress perturbs neonatal iron homeostasis in a sex specific manner. Scientific Reports 12, 9341 (2022), doi.org/10.1038/s41598-022-13633-z.
-
Sarkar, P. et al. Detection of Maternal and Fetal Stress from ECG with Self-supervised Representation Learning. Scientific Reports 11, 24146 (2021), doi.org/10.48550/arXiv.2011.02000.
-
Antonelli, M.C. et al. Early Biomarkers and Intervention Programs for the Infant Exposed to Prenatal Stress. Current Neuropharmacology 20(1), 94–106 (2022), https://doi.org/10.2174%2F1570159X19666210125150955.