How long-distance travel ends and diversity emerges
The last years have taught us many things – and the last one especially, has made us aware that you cannot take long-distance interactions for granted and that diversity is greatly underappreciated. Both long-distance interactions and diversity are key to the mission of the TUM-IAS – and also happen to be at the core of the questions that the TUM-IAS Focus Group Subcellular Dynamics in Neurons has addressed over the past few years.


Focus Group Subcellular Dynamics in Neurons
Prof. Maya Schuldiner (Weizmann Institute of Science), Alumna Hans Fischer Senior Fellow | Prof. Melike Lakadamyali (University of Pennsylvania), Alumna Hans Fischer Fellow | Caroline Fecher, Antoneta Gavoci, Natalia Marahori, (TUM), Doctoral Candidates | Host: Prof. Thomas Misgeld, Neuronal Cell Biology, TUM
Composed of one super-resolution microscopy expert and cell biologist (Melike Lakadamyali), one systems biologist and geneticist (Maya Schuldiner), and one neuroscientist (Thomas Misgeld), this Focus Group set out to explore how nerve cells regulate the life of their functional compartments, called organelles (“small organs”).
Imagine a nerve cell: Its metabolic center is a small cell body, perhaps 50 micrometers in diameter, that has some shorter processes (a few hundred micrometers – called dendrites) and one really long one (up to many centimeters long – the axon), studded with small communication centers, called synapses. Each of these parts of a neuron represents a specific compartment with a specialized function and correspondingly specialized metabolic and energy demands. Nerve cells – like almost all cells – derive most of their energy from a set of organelles called mitochondria, that can exist in a dynamic network or as a set of rather discrete bean-shaped structures. In order to ensure the right number of functional mitochondria in all its compartments, the nerve cell entirely depends on long-distance transport of organelles (likely often lasting days or even weeks), but also utilizes a complex system of local biogenesis, anchorage, and degradation. Beyond the trope of mitochondria as the “power house” of cells, these fascinating organelles do many more things than provide energy – they are involved in numerous biosynthetic pathways, contribute to regulating ionic balances in cells, and can even initiate and regulate cell death (arguing that from a cell’s perspective, mitochondria can turn quickly from energy provider to “hazardous goods”). So how does a single organelle fulfill so many tasks? And how is this regulated to match the specific needs of the different parts of a nerve cell? How is mitochondrial homeostasis (“mitostasis”) – i.e., the right number of mitochondria at the right place – ensured in neurons?
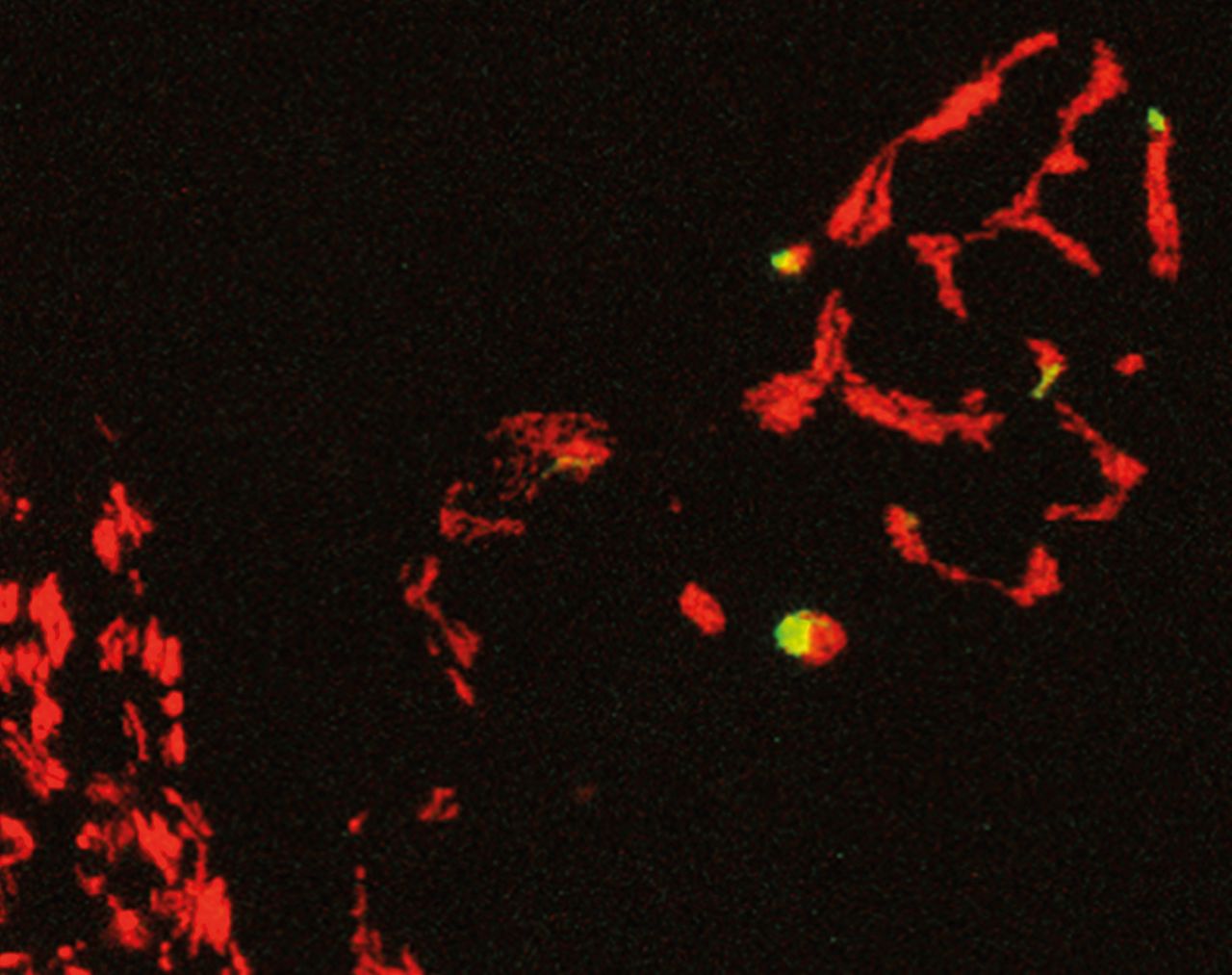
The Focus Group Subcellular Dynamics in Neurons has worked on these questions over the past few years in a two-pronged approach. To address the question of mitochondrial distribution, we took advantage of Melike Lakadamyali’s unique expertise in using fluorescent dyes that can switch their color when exposed to a brief flash of ultraviolet light. This makes it possible to label a small subset of mitochondria anywhere in a nerve cell (Figure 1) and follow their further fate (provided one has access to the right microscopes and model organisms, as Thomas Misgeld’s lab provided). Such an optical “pulse chase” experiment has allowed us to do precise accounting of the flow of mitochondria through a nerve cell's processes. What we discovered is that instead of shipping “spent” mitochondria all the way back from the distant synapses, a nerve cell contains many local degradation sites in synapses, where the cell disposes of a substantial fraction of mitochondria. Moreover, we were able to identify the degradation process as molecularly different from the previously described degradation pathways – an exciting finding, as many of the genes involved are mutated in forms of neurodegeneration, but questions such as how they play together and where they exert their action are not well understood. So while this part of our work revealed that long-distance travel is not always necessary (at least for disposal of cellular junk), another part of our work emphasized the prevailing importance of mitochondrial diversity.
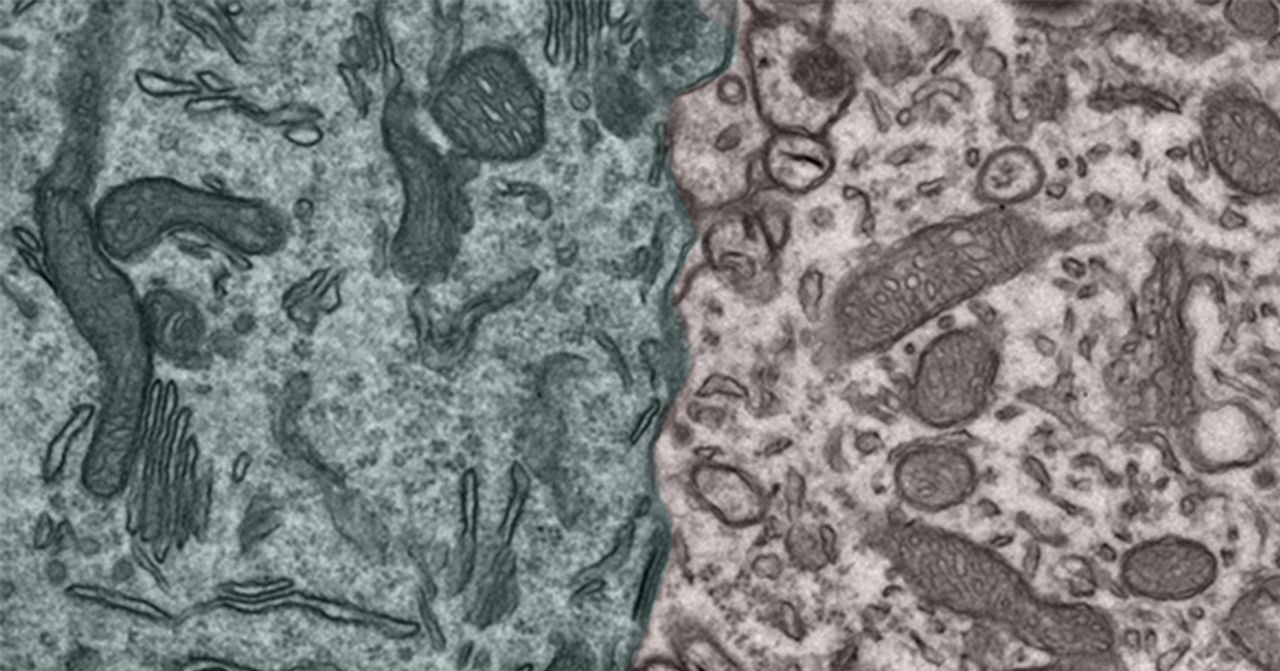
Maya Schuldiner, as a systems-level biologist and geneticist working on organelle biology in yeast, brought a new perspective to the Misgeld lab: Why look at one thing, if there are methods to look at many? So deviating from the Misgeld lab’s traditional approach of pushing the frontier of resolution, we turned toward developing systematic tools to characterize how different mitochondria are in different contexts. Obviously such diversity can exist in shape, in molecular composition, and in function, but also in neighborhood relationships, as many organellar functions are not performed by soloists, but by smal ensembles of different organelles that are joined by so-called “organellar contact sites” (Figure 2). So on the basis of this inspiration, we developed a new and systematic approach to study mitochondrial diversity. In a large consortial effort involving several TUM research groups, we generated a new mouse line that allows us to “tag” mitochondria in any cell type we choose. Using a purification approach that depends on miniature magnetic particles to fish the tagged mitochondria out of tissue homogenates, we can to now determine the molecular composition and functional capacities of cell type- or disease-stage specific mitochondria. We are currently expanding this approach to investigate the differences of mitochondria within cells – where especially in neurons structural changes are well appreciated (e.g., between dendrites and axon), but functional differences are largely elusive.
The Focus Group and its members’ efforts led to a number of distinctions over the course of the Fellowships: Maya Schuldiner became a full professor at the Weizmann Institute of Science in 2019; in the same year, she received her third ERC Grant. In 2020, she was elected to be a member of the German National Academy of Sciences Leopoldina. Thomas Misgeld received TUM's Heinz Maier-Leibniz medal in 2018. In 2018, the Munich Cluster of Systems Neurology was renewed under Thomas Misgeld’s co-speakership (with Maya Schuldiner and Melike Lakadamyali named as Cluster-affiliated TUM-IAS-Fellows of the first funding period). In 2018/19, the MSc “Biomedical Neuroscience” was funded by the Elite Network Bavaria with Thomas Misgeld as a speaker – the program has greatly benefited from teaching input by lecturers at the Weizmann Institute of Science and the University of Pennsylvania, especially during the Covid-19 pandemic.
In summary, the TUM-IAS Focus Group Subcellular Dynamics in Neurons has been able to substantially advance the understanding of how a neuron’s mitochondria regulate their mitochondrial homeostasis and how mitochondrial diversity is created in the nervous system. This work laid the foundation for numerous future collaborative efforts between the Misgeld lab at TUM and the Fellows' labs and their home institutions.
[1]
C. Fecher, L. Trovò, S. A. Müller, N. Snaidero, J. Wettmarshausen, S. Heink, O. Ortiz, I. Wagner, R. Kühn, J. Hartmann, R.M. Karl, A. Konnerth, T. Korn, W. Wurst, D. Merkler, S.F. Lichtenthaler, F. Perocchi and T. Misgeld, “Cell type-specific profiling of brain mitochondria reveals functional and molecular diversity”, Nature Neuroscience, vol. 22, pp. 1731-1742, 2019.
[2]
M. Wang, T. Kleele, Y. Xiao, G. Plucinska, P. Avramopoulos, S. Engelhardt, M.H. Schwab, M. Kneussel, T. Czopka, D. L. Sherman, P. J. Brophy, T. Misgeld and M.S. Brill, “Completion of neuronal remodeling prompts myelination along developing motor axon branches”, Journal of Cell Biology, vol. 220, no. 4, pp. e201911114, 2021.
[3]
N.A. Marahori, M. Schifferer, B. Plomer, T. Kleele, P. Avramopoulos, S. Hannan, S. Engelhardt, M. Lakadamyali, M.S. Brill and T. Misgeld, “A retrograde transit filter that removes synaptic mitochondria,” in preparation.